Nitrogen
Nitrogen is one of the chemical elements in the periodic table; whose chemical symbol is N and its atomic number is 7. Nitrogen is usually in the form of a gas, non-metal, inert, colorless, tasteless and odorless diatom, which covers 78% of the earth's atmosphere and is the main element in living tissues. Nitrogen forms important compounds such as ammonia, nitric acid and cyanides. The human body contains about 3% nitrogen by mass, which is the fourth most abundant element in the body after oxygen, carbon and hydrogen.
Many important industrial compounds such as ammonia, nitric acid, organic nitrates (propellants and explosives) and cyanides contain nitrogen. The extremely strong triple bond in elemental nitrogen (N≡N), the second strongest bond in any diatomic molecule after carbon monoxide (CO), [5] dominates nitrogen chemistry.
It was first discovered and isolated by the Scottish physician Daniel Rutherford in 1772. Although Carl Wilhelm Schiele and Henry Cavendish had independently done so around the same time, Rutherford is generally credited as having published his work first. The French chemist Jean-Antoine-Claude Chaptal proposed the name nitrogen in 1790, when it was found that nitrogen is present in nitric acid and nitrates. Antoine Lavoisier instead suggested the name azote from the ancient Greek: "without life", because it is a suffocating gas.
Nitrogen cycle
Nitrogen is a non-metal group and has a negative electron charge of 3.0. Nitrogen has five electrons in its shell and as a result is trivalent in most compounds. Pure nitrogen is an inert and colorless gas and occupies 78% of the earth's atmosphere. It freezes at 63K and turns into a liquid at 77K, becoming the famous cryogen.
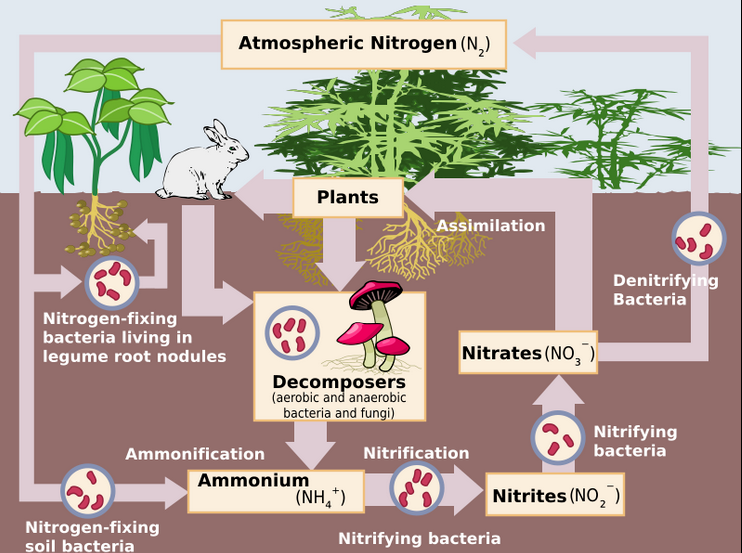
In general, the nitrogen cycle in simple terms consists of: nitrogen in the air turns into nitrogen oxide during lightning, dissolves in the rain, and is absorbed by the soil; in the soil, bacteria into substances that are absorbed by plants convert these compounds. Animals enter nitrogen into their bodies by eating plants, and when animals die and decompose, nitrogen enters the air again.
Plants produce oxygen, energy and glucose (simple sugar) by carrying out photosynthesis. Now, in order for the plant to make protein with the help of sugar, it needs nitrogen, but it cannot receive this nitrogen directly from the air, but its roots in the form of a water-soluble substance called nitrate must absorb it.
History
Nitrogen compounds have a very long history; Herodotus knows ammonium chloride. They were well known in the middle Ages. Alchemists knew nitric acid as aqua fortis (strong water) as well as other nitrogenous compounds such as ammonium salts and nitrate salts. The mixture of nitric and hydrochloric acids was known as aqua regia (royal water), famous for its ability to dissolve gold, the king of metals.
The discovery of nitrogen is attributed to the Scottish physician Daniel Rutherford in 1772, who called it noxious air.
The first military, industrial, and agricultural uses of nitrogen compounds used saltpeter (sodium nitrate or potassium nitrate), especially in gunpowder and later as fertilizer.
Applications
The applications of nitrogen compounds are naturally very diverse due to the enormous size of this class: hence, only applications of pure nitrogen are considered here. Two-thirds (2/3) of the nitrogen produced by the industry is sold as gas and the remaining one-third (1/3) as liquid.
Whenever the oxygen in the air poses a risk of fire, explosion or oxidation, this gas is mostly used as an inert atmosphere. Some examples are:
• As a modified atmosphere, pure or mixed with carbon dioxide, to nitrogenate and preserve the freshness of packaged or bulk foods (by delaying rancidity and other forms of oxidative damage).
• In incandescent lamps as a cheap alternative to argon.
• In fire extinguishing systems for information technology equipment
• In stainless steel construction
• About steel hardening by nitriding
• In some aircraft fuel systems to reduce the risk of fire (see inertial system).
• For inflating racing car and airplane tires, reducing inconsistent expansion and contraction problems caused by moisture and oxygen in natural air.
Abundance
Nitrogen is the most abundant element in the Earth's atmosphere by volume. (78%) and for industrial purposes it is obtained by distillation of liquid air. Nitrogen-14 is produced because of nuclear fusion in stars. Nitrogen is one of the main components of animal waste (such as manure or manure) and is usually found in the form of urea, uric acid and compounds of nitrogen products.
Isotopes
Nitrogen has two stable isotopes: (N-14, N-15). The most important of which is the two (N-14 99.634%) that are produced in the CNO cycle in stars. The rest is the N-15 isotope. One in ten artificially produced isotopes has a half-life of nine minutes, and other isotopes have half-lives of a few seconds or less.
Biological-intermediate reactions (such as assimilation, assimilation and incorporation of nitrification) and... Strictly control the dynamics of nitrogen in the soil. These compounds usually cause N-15 enrichment of the bottom layer and drain the product. Of course, this rapid process often includes amounts of ammonium and nitrate as well, because ammonium is preferentially retained by the nitrate canopy. Nitrate soil is better absorbed and combined by tree roots than ammonium soil.
Allotrope of nitrogen
Dinitrogen (N2)
Atomic nitrogen, also known as active nitrogen, is highly reactive, and is a three-radical with three unpaired electrons. Due to the high reactivity of atomic nitrogen, elemental nitrogen usually appears as molecular N2, dinitrogen. This molecule is a colorless, odorless and tasteless diamagnetic gas under standard conditions: it melts at -210°C and boils at -196°C. Dinitrogen is mostly inactive at room temperature, but it reacts with lithium metal and some transition metal complexes. This is because of its bonding, which is unique among diatomic elements in standard terms because it has an N≡N triple bond. The triple bonds have short bond lengths (in this case, 109.76 pm) and high dissociation energy (in this case, 945.41 kJ/mol), and are therefore very strong, which explains the chemical inertness of dinitrogen.
Nitrogen sources
Nitrogen is available from various sources such as industrial fixation, its atmospheric fixation, biological fixation and organic sources; that industrially available nitrogen is the most important source of nitrogen in the world. Caro did the first industrial fixation of nitrogen in the world in 1901 using N2 and Ca (CN2) from calcium carbide. After that, Harber and Bosh produced ammonia from nitrogen gas and hydrogen gas at a high temperature of 400-6000 degrees Celsius and a pressure of about 200-1000 atmospheres.
Atmospheric nitrogen fixation
Atmospheric nitrogen fixation occurs when nitrogen gas (N2) is broken down by light energy, becomes nitric oxide (NO2), and then combines with oxygen to produce nitrate; which is transported to the ground by rain. The amount of nitrogen fixed by this method is small.
Biological fixation of nitrogen
Microorganisms do this method of nitrogen fixation. These microorganisms convert atmospheric nitrogen into ammonium through the ammonification process (by bacteria); And then by bacteria such as nitrosomonas, nitrosospira, and nitrosococcus, ammonium is converted into nitric oxide, and then by bacteria such as nitrobacter, nitrospira, nitrospina, and nitrococcus, nitric oxide is converted into nitrate, which is known as the best absorbable form for plant growth and development (8).
Organic nitrogen fixation
There are different types of organic fertilizers that contain nitrogen. Concentrated fertilizers such as yard manure (0.5% N), chicken manure (3.03% N), farm compost (0.5% N) and green manure (G.M).
Nitrogen production methods
The most important industrial method for hydrogen production is the hydrocarbon catalytic steam process, in which gaseous or vaporized hydrocarbons are processed with high-pressure steam over a nickel catalyst at 650-950 °C to produce carbon oxides and hydrogen.
CnH2n+2 + nH2O → nCO + (2n + 1)H2;
CnH2n+2 + 2nH2O → nCO2 + (3n + 1)H2.
Depending on the intended use of hydrogen, the primary reaction products are processed in different ways. Another important process for hydrogen production is the non-catalytic partial oxidation of hydrocarbons under high pressure:
CnH2n+2 + (n/2)O2 → nCO + (n + 1)H2.
The process requires a feed system to deliver precise rates of fuel and oxygen, specially designed burners to rapidly mix the reactants, a refractory reactor, and a cooling system to recover heat from the exhaust gases. The second process is exothermic (heat production), as opposed to the endothermic (heat absorbing) vapor-hydrocarbon process.
In the third process, called the pressurized catalytic partial oxidation method, the two previous processes are combined to maintain the required reaction temperature without external heating of the catalyst bed. The superheated steam and hydrocarbons are mixed, preheated, and combined with heated oxygen in a diffuser above the catalytic reactor. Oxygen reacts with hydrocarbons in the space above the catalyst. The reactants then pass through a bed of nickel catalyst where steam-hydrocarbon reactions proceed almost to equilibrium.
Reference:
- "Gases - Density". The Engineering Toolbox. Retrieved 27 January 2019.
- Rutherford, Daniel (1772) "Dissertatio Inauguralis de aere fixo, aut mephitico Archived 2020-08-06 at the Wayback Machine" (Inaugural dissertation on the air [called] fixed or mephitic), M.D. dissertation, University of Edinburgh, Scotland. English translation: Dobbin, Leonard (1935). "Daniel Rutherford's inaugural dissertation". Journal of Chemical Education. 12 (8): 370–75.
- "Improvements in the Manufacture of Nitric Acid and Nitrogen Oxides", published .
- Priestley, Joseph (1772). "Observations on different kinds of air". Philosophical Transactions of the Royal Society of London. 62: 147–256.
- Bowden, F. P. (1958). "Initiation of Explosion by Neutrons, α-Particles, and Fission Products". Proceedings of the Royal Society of London A.
- Reich, Murray; Kapenekas, Harry (1957). "Nitrogen Purfication. Pilot Plant Removal of Oxygen". Industrial & Engineering Chemistry. 49 (5): 869–73.
- Kennett, Andrew J. (2008). Design of a pneumatically assisted shifting system for Formula SAE® racing applications (Thesis). Dept. of Mechanical Engineering, Massachusetts Institute of Technology.



